
Plants can plastically respond to light competition in three strategies, comprising vertical growth, which promotes competitive dominance; shade tolerance, which maximises performance under shade; or lateral growth, which offers avoidance of competition. Here, we test the hypothesis that plants can ‘choose’ between these responses, according to their abilities to competitively overcome their neighbours. We study this hypothesis in the clonal plant Potentilla reptans using an experimental setup that simulates both the height and density of neighbours, thus presenting plants with different light-competition scenarios. Potentilla reptans ramets exhibit the highest vertical growth under simulated short-dense neighbours, highest specific leaf area (leaf area/dry mass) under tall-dense neighbours, and tend to increase total stolon length under tall-sparse neighbours. These responses suggest shifts between ‘confrontational’ vertical growth, shade tolerance and lateral-avoidance, respectively, and provide evidence that plants adopt one of several alternative plastic responses in a way that optimally corresponds to prevailing light-competition scenarios.

Competition plays a fundamental role in plant ecology, but in many habitats the occurrence of neighbours can vary over space and time and plants have evolved both the ability to detect the presence of neighbours and to plastically adjust their phenotypes in response to it 1,2,3,4,5 . In particular, competition for light is known to elicit two types of well-studied plastic responses in plants, known as shade avoidance and shade-tolerance 2, 6,7,8 . Shade-avoidance responses comprise a suite of morphological adjustments, such as enhanced elongation of the stem or petioles, and their increased vertical inclination (i.e. vertical angle). These responses result in vertical growth, thereby allowing plants to position their leaves in favourable high-light conditions 2, 3, 6, 7, 9, 10 . Shade-avoidance responses are elicited in plants via cues indicating neighbour proximity, including the reduction in photosynthetically active radiation (PAR) and in the ratio of red to far-red wavelengths (R:FR) 7, 9, 11,12,13 , as well as mechanical stimulation or plant volatiles 12, 14, 15 . Although termed ‘shade avoidance’, vertical growth does not necessarily provide avoidance of competitive interactions, particularly in dense stands where plants might engage in a competitive arms race for light 16, 17 . Instead, vertical elongation could be depicted as a ‘pre-emptive or confrontational’ behaviour by which plants can deprive each other of light and gain a competitive advantage 18 .
Shade-tolerance responses consist of several well-studied morphological and physiological adjustments that promote plant performance under limited light conditions 6, 8 . Such tolerance is achieved by increasing light-capture efficiency, e.g. by increasing leaf area at the expense of leaf thickness, which results in increased leaf area to leaf mass ratio (specific leaf area) 6, 8, 19, 20 . Shade tolerance can also be achieved by adjusting photosynthetic responses, e.g. by decreasing photosynthetic capacity 6, 8, 20 . Unlike shade-avoidance responses, which are induced by cues specifically indicating neighbour proximity, shade-tolerance responses are known to be elicited in plants mainly via decreases in PAR levels 8, 19 .
In addition, some plants can respond to light-competition cues by employing competition-avoidance behaviours that could improve light intercept and minimise competitive interactions, e.g. by growing away from their neighbours 10, 18, 21 . This response is particularly suitable for procumbent plants, which can grow horizontally 21 , but even more so for clonal plants, which can ‘move’, e.g. by increasing internode length of their stolons or rhizomes, thus actively positioning new ramets in less crowded patches 10, 22,23,24 .
Plants, and clonal plants in particular, can therefore employ three categories of response to light competition: vertical ‘confrontational’ growth, shade tolerance or lateral avoidance 8, 18, 25, 26 . Previous studies have examined the different evolutionary backgrounds that might select for either one of these responses, such as the canalisation of shade tolerance in tropical woody species 27, 28 . Other studies have shown that in habitats where light competition is extremely strong, such as in forest understories, plants are less responsive to light competition cues in their vertical elongation 29,30,31,32,33 . However, even within the same habitat, the strength of competitive interactions imposed on plants can unpredictably vary in both space and time due to stochastic processes such as dispersal or environmental heterogeneity, and plants can encounter neighbours with different stature, age or density. Plants from such heterogeneous environments would therefore benefit from being able to (a) perceive the competitive ability of their neighbours relative to their own and (b) ‘choose’ between the three response categories according to the probable fitness outcome of each 18, 26 . Accordingly, Novoplansky 18 proposed that plants are likely to invest in vertical growth when growing among equal-sized competitors, but when challenged by taller competitors that are less likely to be outgrown, plants are expected to shift to either shade tolerance or, in the case of procumbent or clonal plants, to avoidance responses via horizontal spread.
Previous studies have provided evidence that plants can assess the size of their neighbours and adjust their vertical elongation accordingly 34,35,36,37 . Additionally, plants can refrain from vertical elongation when facing kin neighbours with which they can cooperate 13 , or when confronted with neighbours they are less likely to outcompete, such as trees 23, 38, 39 . If plants can thus evaluate the competitive ability of their neighbours, it is likely that they can not only adopt the optimal strategy within a certain response (e.g. extant of vertical elongation) but also between different responses to competition. Namely, they could shift to alternative plastic responses that confer either tolerance or avoidance of light competition, thus tailoring their response to prevailing and future competitive circumstances 18, 26 . However, to the best of our knowledge, this hypothesis has not been previously tested.
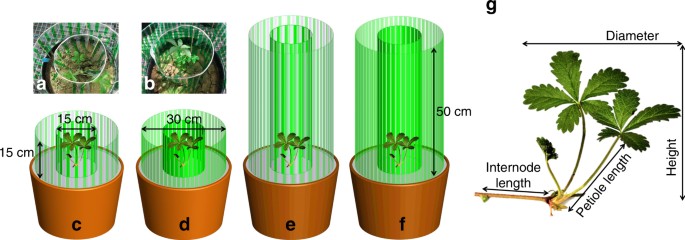
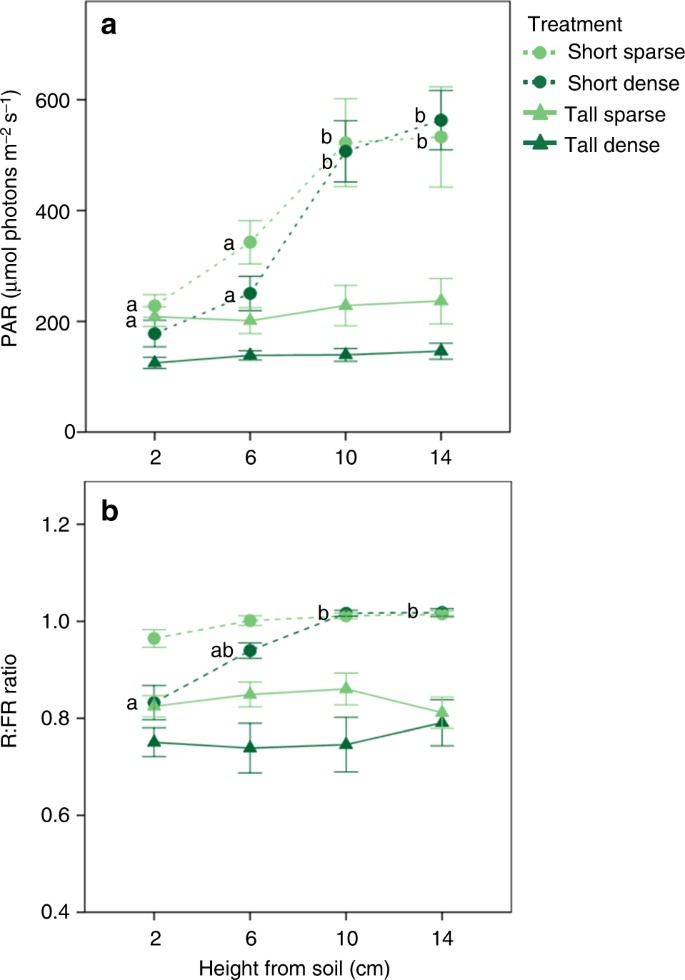
Here, we examined the ability of plants to shift between these three alternative plastic responses according to their competitive environment. We used an experimental setup with the clonal plant Potentilla reptans that simulated, using vertical stripes of transparent green plastic filters, different light-competition settings, by changing both the height and density of the surrounding vegetation. This setup provided a realistic simulation of light competition due to the use vertical filters, which created gradients in both PAR and R:FR particularly in the short treatments. Such use of vertical filters has been shown to induce greater competitive responses compared to homogeneous horizontal shade 23, 40 . Moreover, the use of spaced filter stripes provided plants the possibility to express both vertical and horizontal growth in response to competition cues without any confounding effect of neighbour identity. Here, we predicted that P. reptans would exhibit (1) increased vertical inclination (measured as height-per-diameter ratio) when subjected to treatments simulating similarly sized and dense neighbours, which can be outgrown vertically but present limited advantage of horizontal escape; (2) greater shade tolerance responses (measured as specific leaf area) when subjected to treatments simulating taller, and particularly dense neighbours, which offer limited advantages of both vertical or horizontal growth; and (3) increased lateral avoidance (measured as total stolon length and internode length) when subjected to taller but sparse neighbours, which cannot be outgrown vertically but offer greater light availability in the horizontal direction. Our results corroborate these predictions, suggesting that P. reptans can shift between three different phenotypes, each of which represents alternative optimal plastic response to a different light-competition scenario.
The performance of P. reptans ramets in response to the different treatments, which was measured as the number of newly-produced leaves, was highest under the short-sparse treatment and lowest in the two tall treatments, with intermediate growth under the short-dense treatment (Table 1, Figs. 1a and 2), indicating that plants were negatively affected by simulated competition. In addition, P. reptans exhibited greater petiole length and greater height under the dense compared to the two sparse treatments (Table 1, Figs. 1b and 2). The increased height in the dense treatments is also depicted in their shift further along the height–diameter relationship in the standardised major axis (SMA) test (Table 2, Fig. 3a).
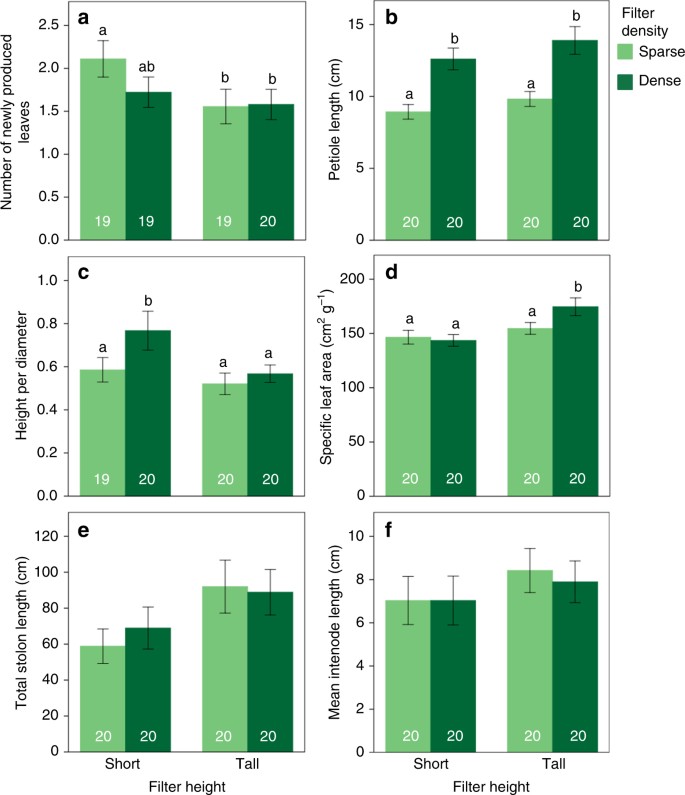
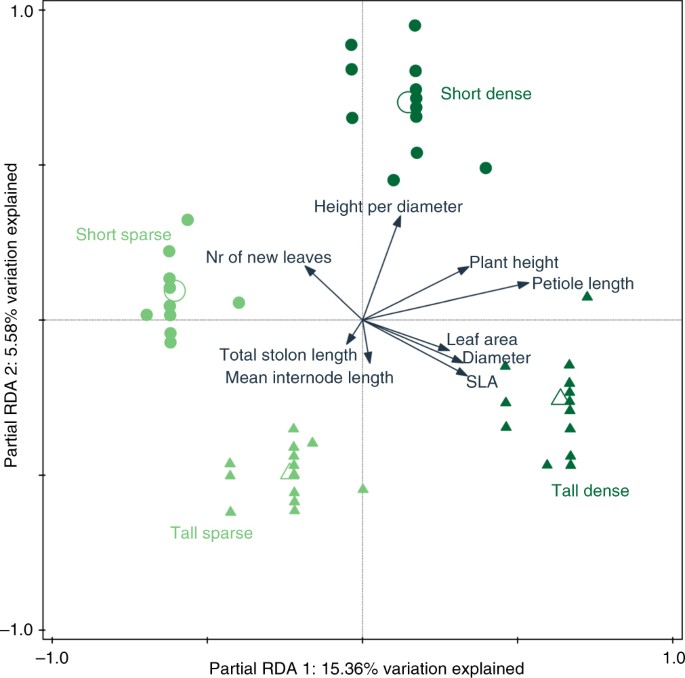
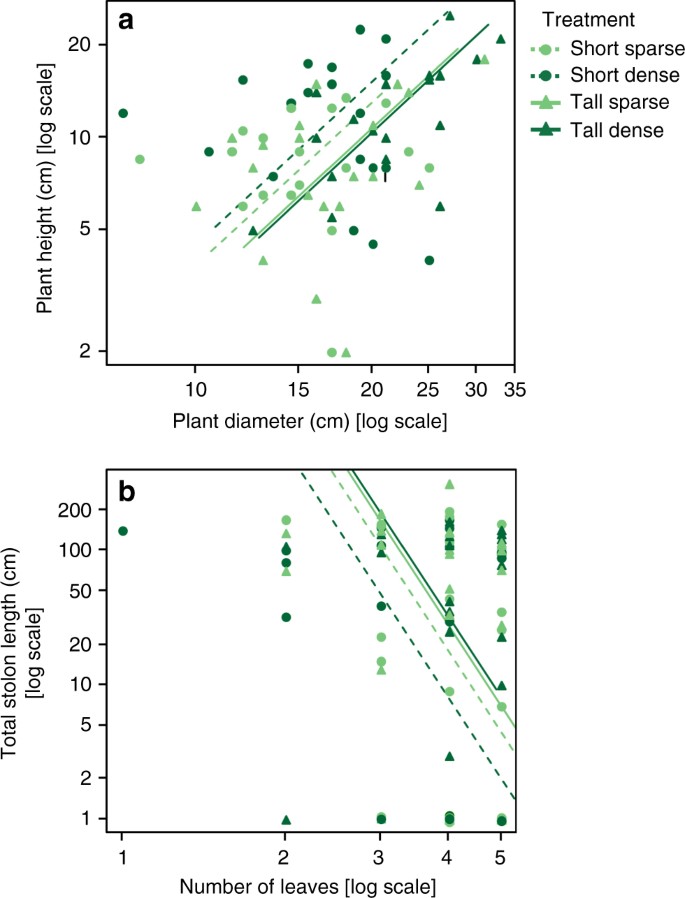
Unlike petiole length and height, the vertical inclination exhibited by P. reptans, which was measured as height-per-diameter ratio, increased not only in response to filter density but also in response to reduced filter height (Table 1). These responses resulted in the greatest vertical inclination when plants were subjected to simulated short-dense neighbours (Figs. 1c and 2; see also Fig. 4a, b for representative photographs of height inclination of plants under the short-sparse vs. short-dense treatments). The greater vertical inclination in response to the short and dense treatments is also depicted in the SMA analysis, where they show greater elevation (y-intercept) and shifts along the slope, respectively (Table 2, Fig. 3a). These results suggest that shifts in plant height in this case were independent of plant diameter. This independence is further confirmed by the right angle between the height-per-diameter and diameter response arrows in the ordination diagram (Fig. 2).
Ramet performance in response to the different treatments was estimated from the number of newly produced leaves per ramet, i.e. number of leaves at the end of the experiment minus number of leaves at its onset. Leaf number has been shown to provide an appropriate non-destructive estimate of plant size in P. reptans 54 , and was also found to highly correlate with ramet biomass in a separate experiment (Supplementary Fig. 1a). The numbers of newly produced leaves in one ramet in the short-sparse, short-dense and tall-dense treatments were lost due to technical problems, resulting in a sample size of 19 per treatment.
Measurements of the plastic responses of P. reptans were carried out in August 2014, 9 weeks after the onset of the experiment. These measurements included petiole length as well as plant height, which was estimated as the vertical distance between the highest leaf tip and the soil surface. Vertical inclination in response to competition was evaluated as the ratio between plant height and its diameter, i.e. the maximum distance between the two furthermost leaf tips. This ratio was chosen because it proved easier to measure within the shading apparatus compared to petioles angles, and due to its high correlation with the latter (Supplementary Fig. 1b). Plant height in one ramet in the short-sparse treatment was lost due to technical problems, resulting in a sample size of 19 for this treatment.
Shade tolerance was estimated with specific leaf area, i.e. the ratio between lamina area and its dry weight. To that end, the two biggest laminas per ramet were harvested and photographed, and their images were used to quantify mean lamina area with the ImageJ software 56 . Laminae’s biomass was measured following oven drying them in 70 ˚C for 3 days. Lateral clonal growth was estimated by measuring total stolon length per plant as well as mean internode length of the stolons.
All variables were measured in the pots prior to the removal of the filters so as to not disrupt plant architecture, except for lamina area and biomass. For the latter variables, samples were identified according to randomly assigned numbers rather than treatment names to blind the investigator to treatment identity. All collected data are available in the Supplementary Data.
The responses of P. reptans to the height and density of simulated neighbours expressed by their number of newly produced leaves, petiole length, height-per-diameter ratio, specific leaf area, total stolon length and mean internode length were examined using a generalised linear-mixed model with filter height and density as fixed factors and plant genotype as a random factor. The number of newly produced leaves, petiole length, height-per-diameter ratio and specific leaf area were analysed with a normal probability distribution with an identity link function. Total stolon length and internode length were analysed with a Gamma probability distribution with a log link function. To account for potential differences in total stolon length due to ramet size 57, 58 , the number of leaves per ramet at the end of the experiment was used as a covariate (after confirming the assumption of homogeneity of slopes). Post hoc pairwise comparisons between treatments were performed using false discovery rate correction for multiple tests 59, 60 . These statistical analyses were performed using PASW 18 (SPSS).
In addition to the univariate analyses, a multivariate approach was employed to evaluate the complete array of plastic responses displayed by P. reptans under the different treatments. Partial redundancy analysis (RDA) was performed with diameter, height per diameter, leaf area, mean internode length, number of new leaves, petiole length, plant height, specific leaf area and total stolon length as response variables explained by the simulated neighbour treatments after removing the effect of covariates (number of leaves at the end of the experiment as an estimate of plant size and genotype). To account for different measuring units, traits were centred and standardised. Monte-Carlo permutation test (n = 999) on first and second RDA axes was used with genotype as permutation block 61 . Furthermore, to evaluate the effect of each of the treatments independently, simple tests for each treatment were performed using the false discovery rate correction for multiple tests 50, 62 . The multivariate analyses were performed using CANOCO 5 63 .
To better estimate the treatment effects on the allometric relationships between plant height and diameter as well as between total stolon length and number of leaves, these relationships and effects were analysed using the SMA regression, which is appropriate for analysing variables that have no causal relationship 64 . SMA was used to test for the effect of filter height and density on shifts along a common slope and on elevation (y-intercept) between slopes (after confirming the assumption of homogeneity of slopes). The traits were log-transformed prior to the analysis. These analyses were performed using the smart package in R 62 .
Data analysed in this study are included in the Supplementary Information
We thank Nadine Bihler, Elena Spöri, Sarah Gebauer, Ricarda Gatter, Peter Armstrong and Lorenz Henneberg for their assistance with the experimental setup; Pierre Liancourt, Jan Ruppert and Udi Segev for valuable discussions and contributions to this work; and Shimon Gruntman for producing the graphic illustrations of the experiment. This study was supported by a grant of the Tübingen Athene Program to M.G.